
-
Raltegravir potassium
CAS No.: 871038-72-1 Formula: C20H20FKN6O5 Molecular Weight: 482.50700Search Suppliers
-
Raltegravir potassium
CAS No.:871038-72-1
Formula:C20H20FKN6O5
Molecular Weight: 482.50700
Description
In December 2011, it received FDA approval for pediatric use in patients ages 2–18, taken in pill form orally twice a day by prescription with two other antiretroviral medications to form the cocktail (most anti-HIV drugs regimens for adults and children use these cocktails). Raltegravir is available in chewable form, but because the two tablet formulations are not interchangeable, the chewable pills are only approved for use in children two to 11. Older adolescents will use the adult formulation.
Basic Info
Chemical Name |
Raltegravir potassium |
|---|---|
Synonyms |
N-[(4-Fluorophenyl)methyl]-1,6-dihydro-5-hydroxy-1-methyl-2-[1-methyl-1-[[(5-methyl-1,3,4-oxadiazol-2-yl)carbonyl]amino]ethyl]-6-oxo-4-pyrimidinecarboxamide Potassium Salt; Raltegravir (potassium salt); MK 0518; Raltegravir K; Raltegravir(MK-0518); Raltegravirpotassium; Expand |
CAS No. |
871038-72-1 |
Molecular Formula |
C20H20FKN6O5 |
Molecular Weight |
482.50700 |
PSA |
155.07000 |
LogP |
2.13150 |
Numbering system
| UNII | 43Y000U234 |
|---|
Properties
Appearance & Physical State |
Off-white solid |
|---|---|
Density |
1.46 g/cm3 |
Melting Point |
282ºC |
Storage Condition |
-20ºC Freezer |
Safety Info
HS Code |
2934999090 |
|---|

Other items you might be interested in
-
CAS No.: 112945-52-5
Silica, fumed
-
CAS No.: 33069-62-4
Paclitaxel
-
CAS No.: 11021-13-9
Ginsenoside Rb2
-
CAS No.: 26328-04-1
Cinepazide maleate
-
CAS No.: 501-36-0
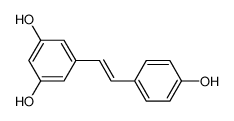
Resveratrol
-
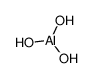
CAS No.: 21645-51-2
Aluminium hydroxide









-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
-
-

-
More Suppliers>>Boc Sciences
UNITED STATES
Purity: 98%
Lead Time: 1 Week(s)
Price: Min $269 /g
Changzhou Xinxinglian Biotechnology Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Wenzhou Win-Win Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 3 Day(s)
Price: -
Hangzhou J&H Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Xiamen Zhixin Chemical Co., Ltd.
CHINA
Purity: 99%
Lead Time: 3 Day(s)
Price: -
CHINA
Purity: 99%
Lead Time: 14 Day(s)
Price: -
Henan Coreychem Co.,Ltd
CHINA
Purity: 98%
Lead Time: 2-3 Day(s)
Price: -
Hangzhou DayangChem Co., Ltd
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: -
Hangzhou Shangjie Chemical Co., Ltd.
CHINA
Purity: 98%
Lead Time: 7 Day(s)
Price: -
Skyrun Industrial Co., Limited
CHINA
Purity: 99%
Lead Time: 7 Day(s)
Price: -