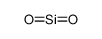
-
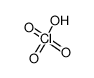
perchloric acid
CAS No.: 7601-90-3 Formula: ClHO4 Molecular Weight: 100.45900
-
perchloric acid
CAS No.:7601-90-3
Formula:ClHO4
Molecular Weight: 100.45900Suppliers: All(0) China Suppliers(0) Price Available(0) Contractor(0)
MSDS
-
Please login ,
and view Chemical MSDS Detail 

Other items you might be interested in
-
CAS No.: 112945-52-5
Silica, fumed
-
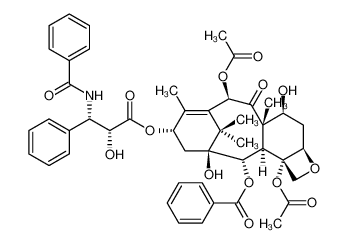
CAS No.: 33069-62-4
Paclitaxel
-
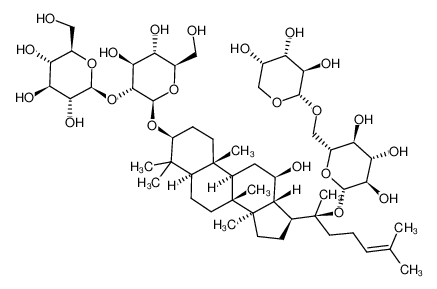
CAS No.: 11021-13-9
Ginsenoside Rb2
-
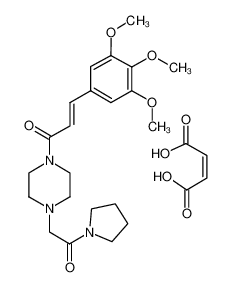
CAS No.: 26328-04-1
Cinepazide maleate
-
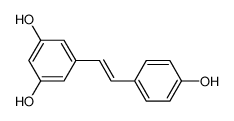
CAS No.: 501-36-0
Resveratrol
-
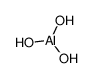
CAS No.: 21645-51-2
Aluminium hydroxide