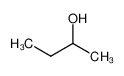
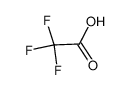
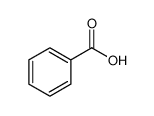
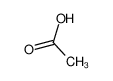
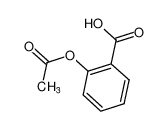
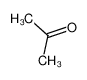
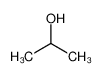
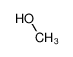
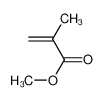
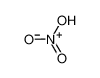
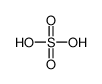
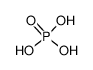
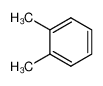
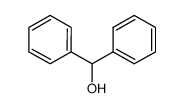
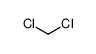
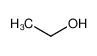
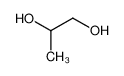
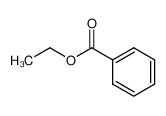
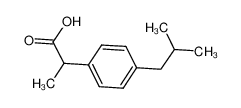
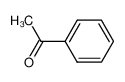
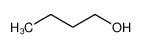
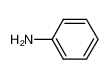
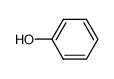
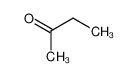
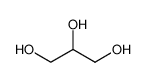
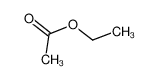
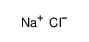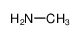-
60,000,000+
Unique chemical entries
-
240,000+
Chemical safety information articles
-
1,600,000+
Synthesis routes and reference documents
-
2,500,000+
Up-to-date SDS and MSDS
-
2,000,000+
NMR references (both 1H-NMR and 13C-NMR)
-
70,000+
Transportation and warehousing regulations and IMDG codes
-
1,000,000+
Downstream products and supplier information
-
200,000+
Compounds updated weekly

Hot Chemical
Biochemicals and Pharmaceutical Chemicals
View MoreMaterial Chemicals
View MoreElectronic Chemicals
View MorePaints and Coatings
View MoreNatural Products and Extracts
View MoreAgrochemicals
View MoreCommodity Chemicals
View MoreCatalysts and Additives
View MoreDyes and Pigments
View MoreFood and Feed Additives
View MoreFlavors and Fragrances
View MoreHousehold Chemicals
View More